Featured
- Get link
- X
- Other Apps
Condensation Reaction Biology Example
Condensation Reaction Biology Example. Taking ethyl acetate (ethyl ethanoate) as an example, claisen condensation reaction involves following steps: In our example above, disaccharide forms, meaning two.
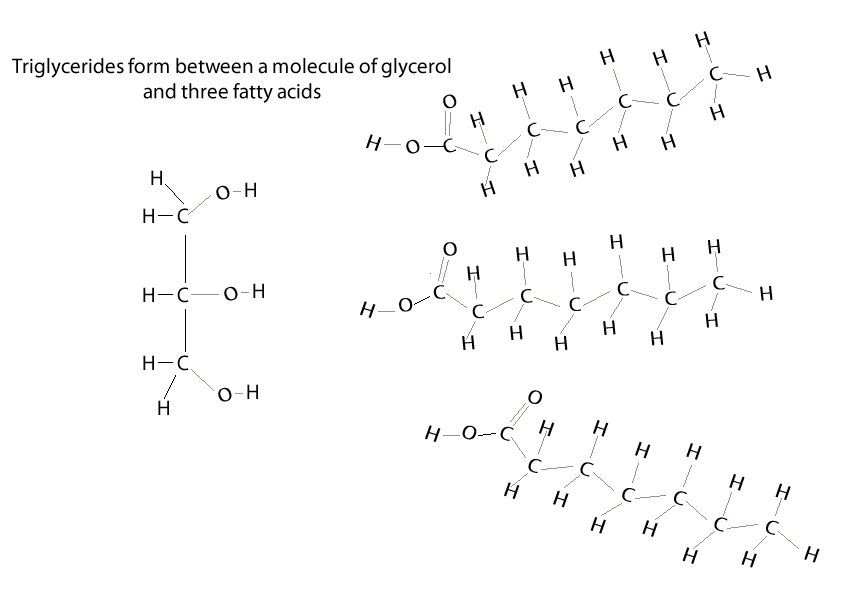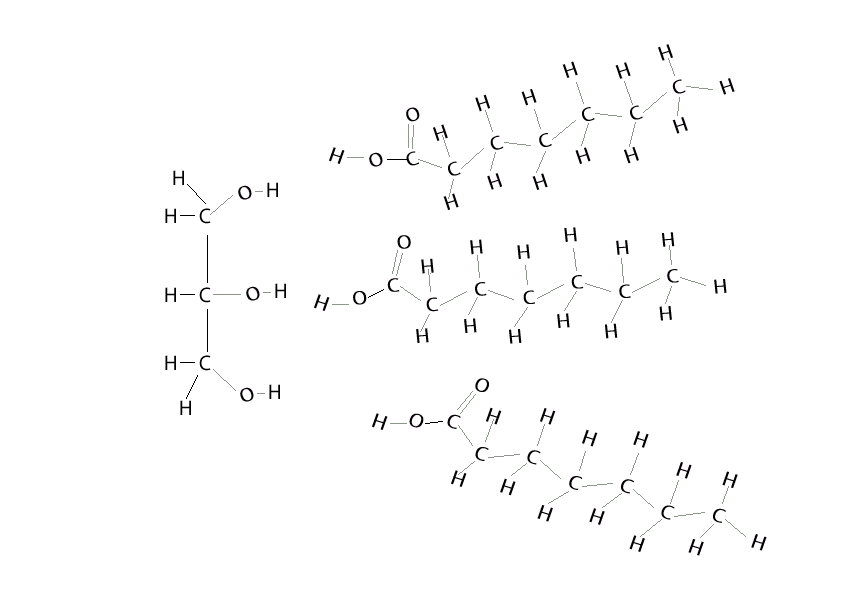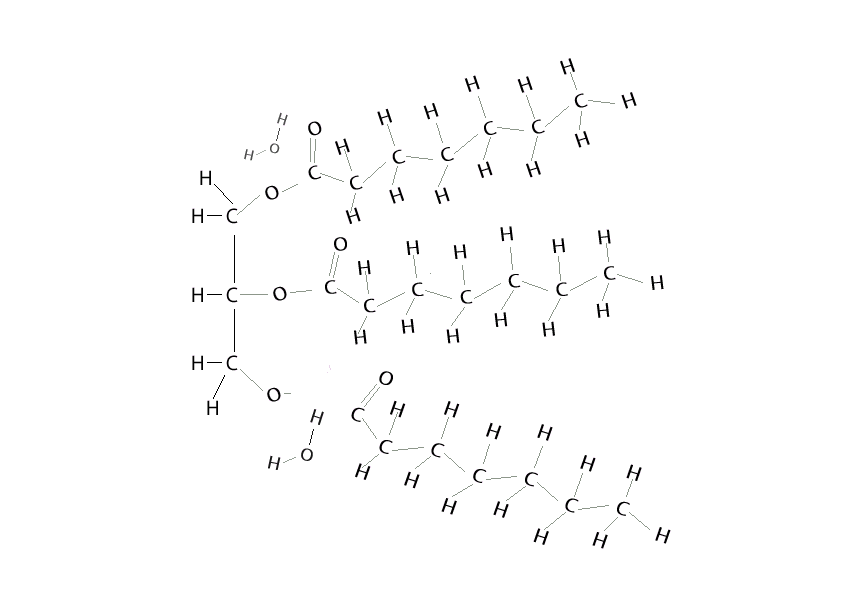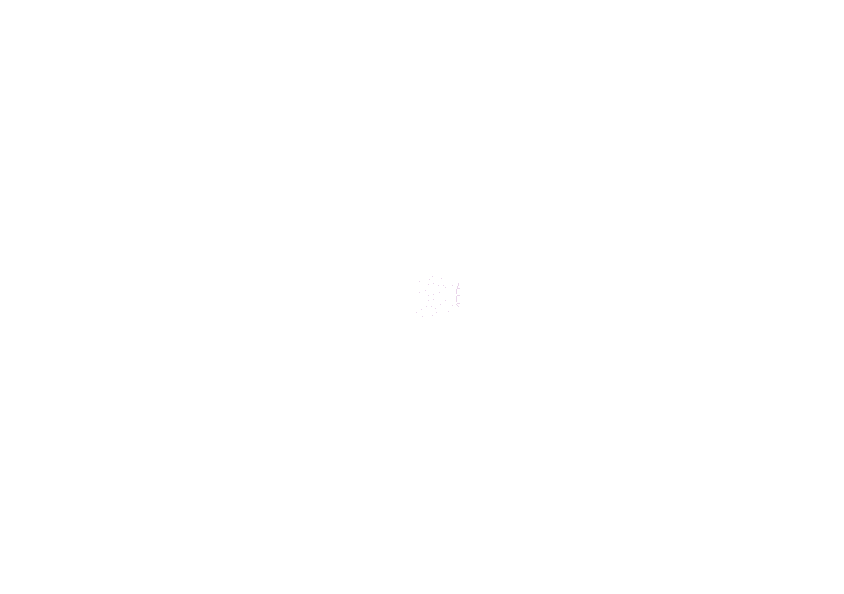
It is the basis for the synthesis of all the. Having a cold soda on a hot day, the can sweats. water molecules in the air as a vapor hit the colder surface of the can and turn into liquid water. Condensation is when a gas turns to a liquid.
Condensation Polymers Are Polymers Made In A Condensation Reaction Between Two Smaller Monomers, Releasing A Small Molecule Called A Condensate In The Process.
Mechanism of claisen condensation reaction. Condensations have been defined to include those reactions in which two molecules are joined with loss of water. A fundamental example of condensation is a cold water bottle.
A Condensation Reaction Is A Reaction In Which One Or More Water Molecules Are Lost From One Or More Reactants (Condensation Is The Reverse Of.
A condensation reaction is a chemical reaction between two compounds where one of the products is water, ethanol, acetic acid, hydrogen sulfide, or ammonia. In the figure given above, there is a. Condensation is responsible for the water inside the frame on a cold day.
Having A Cold Soda On A Hot Day, The Can Sweats. Water Molecules In The Air As A Vapor Hit The Colder Surface Of The Can And Turn Into Liquid Water.
In organic chemistry, a condensation reaction is a type of chemical reaction in which two molecules are combined to form a single molecule, usually with the loss of a small molecule. The water vapors for condensation may originate from your own mouth or maybe rising from the coffee, soup, or. A condensation reaction is a chemical reaction of two different or identical compounds combined to form a single and large mixture of compounds.
Condensation Is Defined As The.
In this video, she will look at how larger molecules are formed from smalle. Condensation is when a gas turns to a liquid. Liz looks at condensation and hydrolysis reactions for your a level biology exam.
Below Is An Example Of A Condensation Reaction Where The Small Molecule Released Is Water, And The New Molecule Formed Is Ab.
Condensation is a chemical process by which 2 molecules are joined together to make a larger, more complex, molecule, with the loss of water. Making polyesters polyesters occur naturally as biological. A simple example is the condensation of two amino acids to form a peptide and the digestion of food is an example of hydrolysis, as water helps to break.
Comments
Post a Comment